The Role of Wnt signaling in Epithelial to Mesenchymal Transition
WNT4EMT
In the proposed research project we aim to determine the involvement of Dishevelled and TCF/LEF families of human genes in the process of Epithelial-to-Mesenchymal Transition (EMT) in brain tumors. We believe that the changes of structure and expression of the selected genes correlate with the phenotypic changes of tumor cells. EMT is a process very much involved in invasion and progression of tumors. Many oncogenic signaling pathways can induce EMT. The classical Wnt pathway has a particularly tight link with EMT and it has been shown that nuclear translocation of beta-catenin can induce EMT. Wnt pathway is one of the basic cellular pathways whose misregulation plays important roles in tumorigenesis and whose mediators of transcription are members of TCF/LEF family. Another important protein family is the Dishevelled considered to be the central hub of Wnt signaling since it interacts with Wnt receptors and recruits the multiprotein beta-catenin destruction complex. The experiments of the proposed research will use modern methods of molecular biology for the analyses of brain tumor samples of different malignancy grades. The genetic changes will be tracked by PCR/loss of heterozygosity, heteroduplex and Spreadex electorphoresis (Elchrom Scientific, Switzerland) methods. Expression of selected proteins will be studied by immuno-histochemistry demonstrating their location in tumor cells. Besides its scientific value, results from the proposed project will have an application in medical diagnostics. Identifying changes in molecules responsible for control of cell motility will give us the preconditions for understanding the invasiveness of brain tumors and offer new disease progression markers. Our experimental evidence would also encourage the development of therapies that specifically interfere with Wnt signaling in cancer.
INFORMACIJE O PROJEKTU
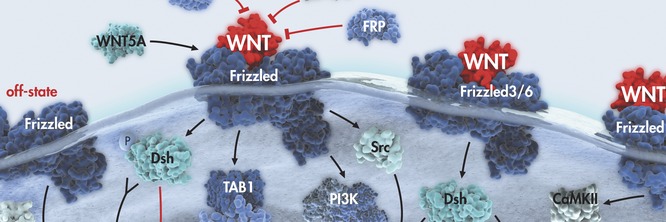
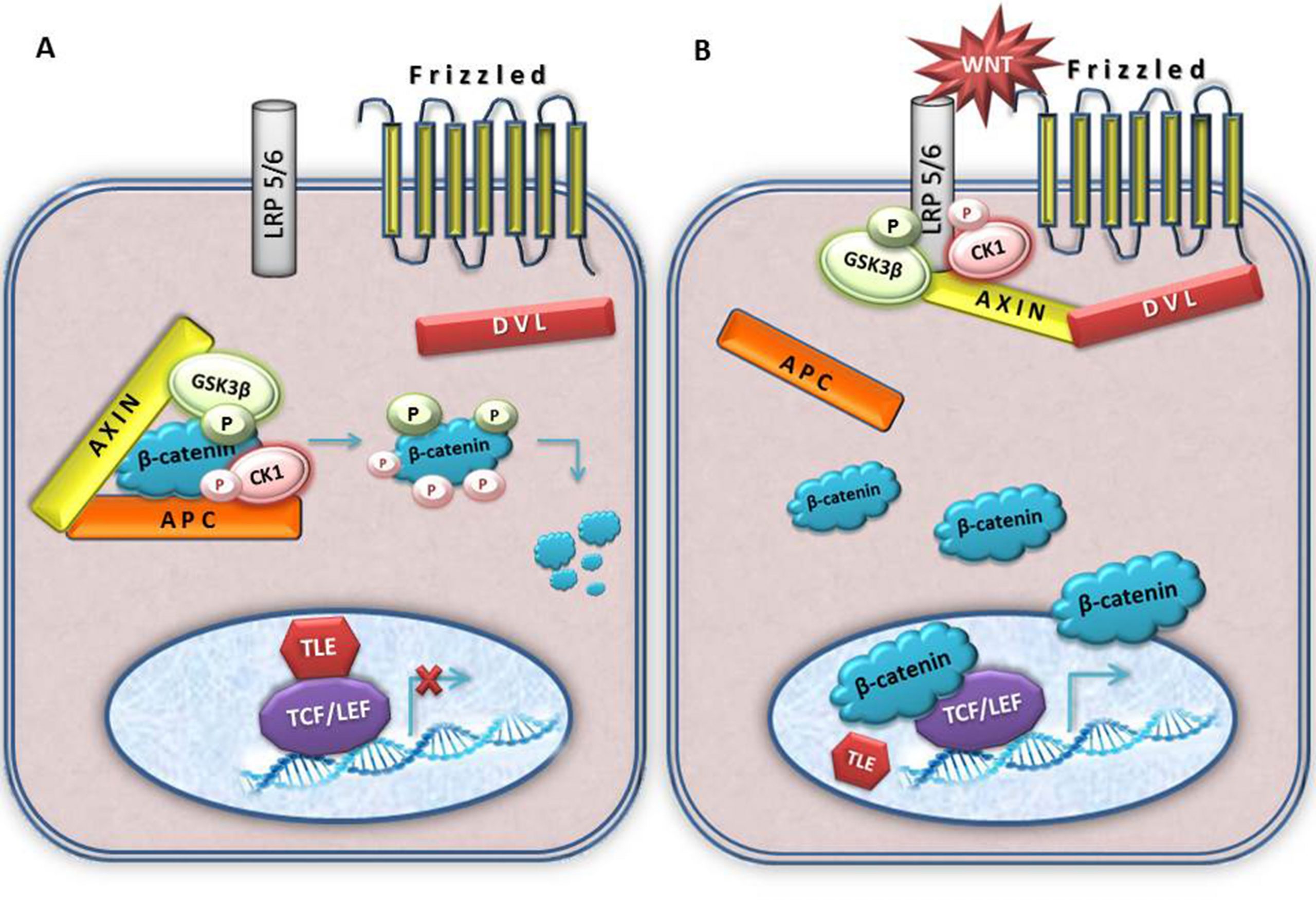
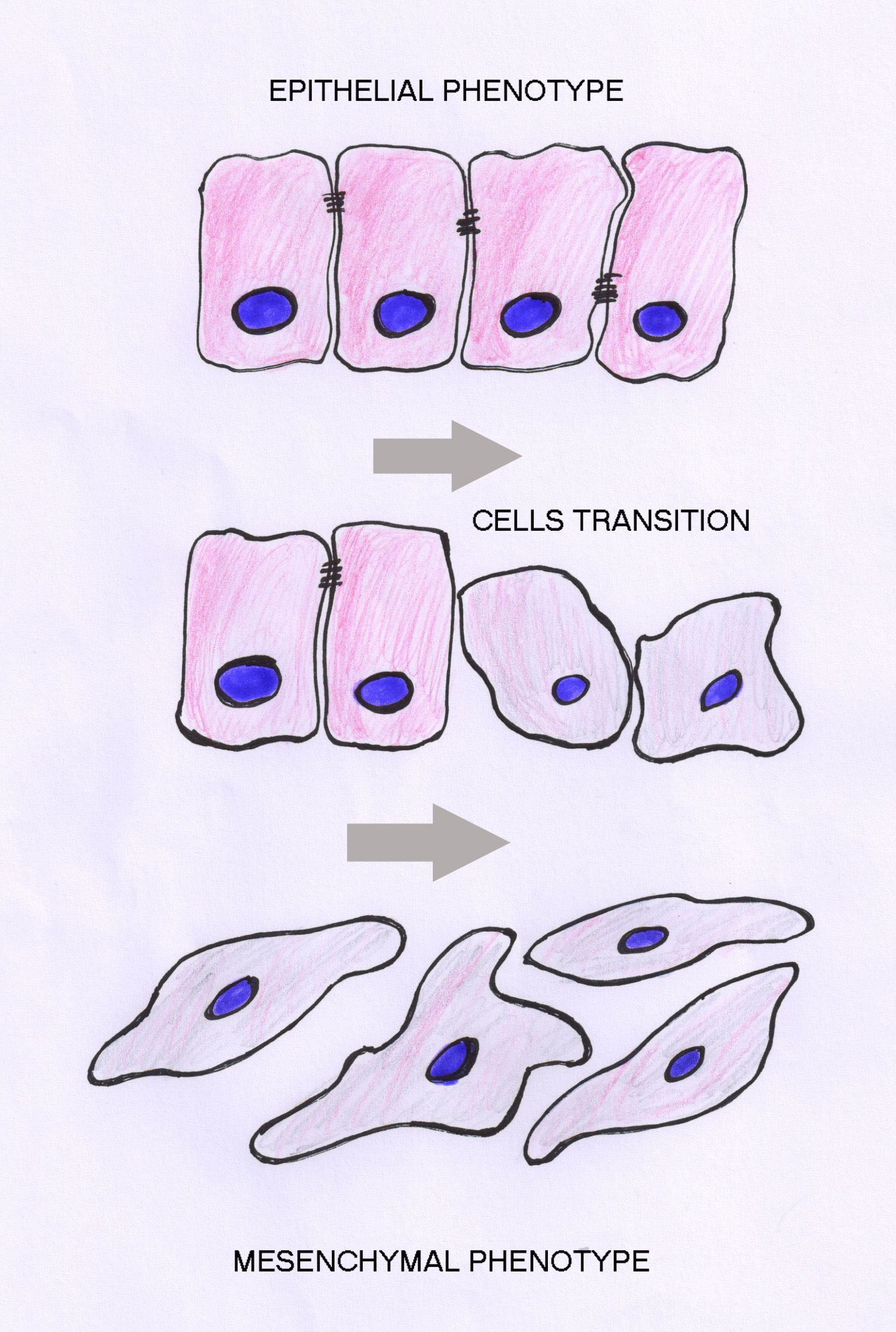
In the proposed research we aim to determine the involvement of Wnt signaling in the process of epithelial-to-mesenchymal transition (EMT) which is responsible for the progression of brain tumors. Our hypothesis is that molecular key components of the wnt pathway play crucial roles in EMT and progression of the central nervous system tumors. We believe that they are changed in invasive cells, and that these changes are reflected in the expression of their protein products. Brain tumors are classified into different grades based on histology and prognosis by WHO. However, despite many recent advances the molecular blueprint of development and progression of brain tumors is still largely unexplained. The formation of mobile cells with invasive potential is the result of multiple consecutive genetic changes that accumulate and represent a critical factor in tumor progression. The full spectrum of signaling agents that contribute to EMT remains unclear. All things considered, EMT is a very complex event requiring the specific spatiotemporal expression of molecules, their interaction and modification of a range of cellular and extracellular factors to allow cellular motility and invasion to proceed. Although brain tumors rarely metastize to distant organs, probably because of blood-brain barrier, their local invasion is the reflection of their motility and invasive behavior.The classical Wnt signaling pathway has a particularly tight link with EMT. Wnt pathway that was discovered 30 years ago has today been established as one of the basic cellular pathways whose misregulation plays important roles in tumorigenesis. Central mediators of transcription of this pathway are members of TCF/LEF (T-cell factor/lymphoid-enhancing factor) transcription factor family. Wnt signaling leads to the stabilization of cytosolic β-catenin. When wnt pathway is activated beta-catenin is translocated to the nucleus, subsequently binding to TCF/LEF family members, which leads to the transcription of Wnt-target genes such as c-myc, matrix metalloproteinase (MMP)-7, cyclin D1, etc. (Novak and Dedhar, Cell Mol Life Sci 56:523-537, 1999; Brantjes et al,Biol Chem 383:255-261,2002; Klaus and Birchmeier, Nat Rev Cancer 8:8387-8398 2008; Shitashige et al, Cancer Sci 99:631-637, 2008; Pecina-Slaus, Cancer Cell Int 10:22, 2010; Cardigan and Waterman, Cold Spring Harb Perspect Biol 2012:4, 2012). In the absence of factors that activate Wnt signaling, the complex APC-Axin and GSK-3 binds to β-catenin with subsequent β-catenin phosphorylation, ubiquination, and degradation by proteasomes. In this scenario TCF/LEF proteins repress target genes through a direct association with co-repressors (Novak and Dedhar, Cell Mol Life Sci 56:523-537, 1999; Brantjes et al,Biol Chem 383:255-261,2002; Stamos and Weis, Cold Spring Harb Perspect Biol, 5:a007898,2013). However, mutations of the complex APC-Axin and GSK-3 can also results in the translocation of beta-catenin to the nucleus ultimately leading to oncogenic transformation and progression. Dishevelled (Dsh or Dvl) is a multifunctional phosphoprotein with 3 members in humans (Dvl-1, Dvl-2 and Dvl-3). Dvl has been shown to shuttle between the cytoplasm and the nucleus. Although its role has not been completely elucidated yet, it is considered to be the central hub of wnt signaling since it is known that it interacts with active wnt receptors and recruits the multiprotein destruction complex. The nuclear translocation of beta-catenin can induce EMT (Wu and Zhou, Acta Biochim Biophy Sin 40:643-648,2008). Besides being the main signaling effector molecule of the pathway, beta-catenin is involved in cellular architecture (Bienz, Curr Biol 15:R64-R67, 2005) too, bound to E-cadherin it is an essential component of adherens junctions. Let me accentuate that the most prominent feature of EMT is the loss of expression of the cell-cell adhesion molecule E-cadherin (Lehembre et al, EMBO J 27: 2603-2615,2008). Moreover, the stabilization and nuclear accumulation of beta-catenin can activate the transcriptional repressors Snail and Slug that suppress E-cadherin expression and thus induce EMT (Wu and Zhou, Acta Biochim Biophy Sin 40:643-648,2008; Li et al, Cancer Lett 336: 379-389,2013). The numerous reports by many authors as well as our own results indicate that E-cadheirn plays a role in CNS tumors – meningiomas (Schwechheimer et al, Virchow Arch 432:163-167, 1998; Pećina-Šlaus et al, J Cancer Res Clin Oncol, 136; 695-702, 2010).
The results of our previous investigations on wnt pathway in human brain tumors demonstrated that our hypotheses were correct. The research conducted within three scientific projects confirmed the important roles of APC, E-cadherin and axin in brain tumor formation (Pećina-Šlaus et al, J. Neurooncol. 87;63-70, 2008; Pećina-Šlaus et al, J Cancer Res Clin Oncol, 136; 695-702, 2010; Nikuševa Martić et al, Pathol Oncol Res16; 75-79; 2010; Pećina-Šlaus et al, Brain Tumor Pathol 28; 223-228; 2011). The relevance of our findings has been recognized by international scientific community and the results of investigations carried out in these projects have been published. A total of 32 scientific papers were published in prestigious international scientific journals. Besides original scientific contributions a book, 40 abstracts, 7 book chapters and numerous professional papers were also published. We presented our work on 27 international meetings. One must not forget the great educational impact of the proposed research. A large number of graduate and postgraduate students express an interest in this type of scientific research as well as a wish to participate in laboratory work related to this subject. Up till now 13 theses were defended and 5 student papers were rewarded Rectors award. The proposed project represents a logical progression of previous research but also opens novel horizons and deepens the scientific problem of Wnt and EMT in brain tumor progression. Elucidating this problem is of primary concern to both medical diagnostics and the development of specific therapies and is, therefore, consistent with strategic priorities of Croatian Scientific Foundation for scientific investigation in Croatia.
Another important purpose of the project is to offer new and more accurate molecular markers of the disease progression, i.e. better diagnostic tools. Our specific goals are the investigation of genes and proteins TCF-1 (TCF7), TCF-3 (TCF7L1), TCF-4 (TCF7L2) and LEF-1 (Cadigan and Waterman, Cold Spring Harb Perspect Biol, 2012;4; Wallmen et al., Nucleic Acids Res, 40; 9455-9469, 2012) and DVL-1, DVL-2 and DVL-3 (Lee et al, Cellular signal, 20: 443-452, 2008; Gao and Chen, Cellular signal, 22: 717-727, 2010; Pulvirenti et al, Cancer Res, 71: 7280-7290, 2011). Finally we would also like to determine the phosphorylation status and cellular location of beta-catenin. For this purpose we will continue to collect samples for the Brain Tumor Bank. In collaboration with University Hospital Sisters of Mercy and University Hospital Center Zagreb we aim to expand (enlarge) and supplement the tumor bank that would encompass different types of brain tumors and to systematize and store all the data in the computer base. Our second aim is to isolate DNA from collected tumor specimen, but also from the collected corresponding blood samples (constitutive DNA). In this way we propose to expand our genomic DNA bank of the collected brain tumors. Our further aim is to analyze genetic changes and genomic instabilities regarding Dishevelled and TCF/LEF families of human genes in collected brain tumors. We will search for genetic instabilities of those genes but would also attempt to detect changes of their protein expression. We plan to analyze gross deletions of tumor suppressor genes by loss of heterozygosity and to detect microsatellite instability (MSI). MSI is the reflection of non-functional cellular mismatch repair systems that is characteristic of tumor cells and represents an aspect of genomic instability characteristic of tumor cells. The research will be carried out at the Laboratory for Neuro-oncology Croatian Institute for Brain Research Medical School University of Zagreb. Modern molecular biology methods are going to be performed using the equipment of the Laboratory for Neuro-oncology and of the Croatian Institute for Brain Research. During the last project period the Laboratory for neuro-oncology at the Croatian Institute for Brain Research has been equipped and all the apparatuses necessary for the proposed experiments are located there. Medical School University of Zagreb and Croatian Institute for Brain Research will ensure the location, facilities infrastructure and administrative services that will enable the project leader and her team to perform the proposed scientific investigations. Medical School University of Zagreb and Croatian Institute for Brain Research will enable the continuing true and realistic support in all the services necessary for conducting the research of the WNT4EMT project.
The importance of proposed scientific field is tremendous. The biggest challenge in brain cancer is the migration of cancer cells. If we could understand the mechanisms of invasion and motility and learn how to control it before malignant cells take off into other parts of the brain, we could find novel molecular targets and improve life expectancy and quality of life.
Identifying changes in molecules responsible for control of cell adhesion and motility will give us the preconditions for understanding the invasiveness of brain tumors, offer new disease progression markers, and thus allow the development of different types of therapies. Besides this short-term and direct use, the achieved results also address long-term considerations related to the development of specific gene therapies for which data collected in this project will supply the foundations.
SUDIONICI PROJKTA
Scientific project leader
Prof. dr. sc. Nives Pećina-Šlaus
Medical School University of Zagreb; Croatian Institute for Brain Research; Laboratory for Neuro-Oncology; Šalata 12, 10 000 Zagreb, nina@mef.hr
Project team members:
Prof.dr.sc. Ljiljana Šerman, dr.med., Zavod za biologiju, Medicinski fakultet Sveučilišta u Zagrebu, Šalata 3, Zagreb, Hrvatska
Prof.dr.sc. Mirna Lechpammer, Department of Pathology & Laboratory Medicine University of California, Davis, Medical Center PATH Building, 4400 V Street, Rm. 1227 Sacramento, CA 95817
Prof.dr.sc. Reno Hrašćan, Zavod za biokemijsko inženjerstvo, Prehrambeno-biotehnološki fakultet Sveučilišta u Zagrebu, Kršnjavoga 20, Zagreb, Hrvatska
Doc.dr.sc. Davor Tomas, Zavod za patologiju Medicinski fakultet Sveučilišta u Zagrebu i Klinički bolnički centar Sestre milosrdnice Vinogradska 29, 10000 Zagreb, Hrvatska
Doc.dr.sc. Tamara Nikuševa Martić, Zavod za biologiju, Medicinski fakultet Sveučilišta u Zagrebu, Šalata 3, Zagreb, Hrvatska
Mag.biol.exp. Anja Kafka, Zavod za biologiju, Laboratorij za Neuroonkologiju, Hrvatski institut za istraživanje mozga Medicinski fakultet Sveučilišta u Zagrebu, Šalata 3, Zagreb, Hrvatska
Prof.dr.sc. Vili Beroš, Zavod za neurokirurgiju, Klinički bolnički centar Sestre milosrdnice, Vinogradska cesta 29, Zagreb, Hrvatska
Dr.sc. Hrvoje Ivan Pećina, Zavod za radiologiju, Klinički bolnički centar Sestre milosrdnice, Vinogradska cesta 29, Zagreb, Hrvatska
Dr.sc. Tomislav Vladušić, Zavod za biokemijsko inženjerstvo, Prehrambeno-biotehnološki fakultet Sveučilišta u Zagrebu, Kršnjavoga 20, Zagreb, Hrvatska
Dr.sc. Martina Zeljko, Zavod za internu medicinu, Sveučilišna bolnica “Merkur”, Zajčeva 19, 10000 Zagreb, Hrvatska
Doc.dr.sc. Goran Mrak, Zavod za neurokirurgiju, KBC Zagreb, Medicinski fakultet Sveučilišta u Zagrebu, Kišpatićeva 12, Zagreb, Hrvatska
Andrej Desnica, dr.med., Klinika za neurokirurgiju, Klinički bolnički centar Zagreb, Kišpatićeva 12, Zagreb, 10000
Anja Bukovac, mag.biol., Laboratorij za Neuroonkologiju, Hrvatski institut za istraživanje mozga Medicinski fakultet Sveučilišta u Zagrebu, Šalata 3, Zagreb, Hrvatska
Prof.dr.sc. Denys Neville Wheatley, Director of BioMedES
FINANCIRANJE
Total budget: 800.000,00 kn
REZULTATI PROJEKTA
Rezultati projekta objavljeni su u časopisima indeksiranim u Web of science bazi
1. Int J Mol Sci 2014, Q2, IF 2,72
Brain Metastases from Lung Cancer Show Increased Expression of DVL1, DVL3 and Beta-Catenin and Down-Regulation of E-Cadherin
2. Histol Histopathol 2014, Q2, IF 2,312
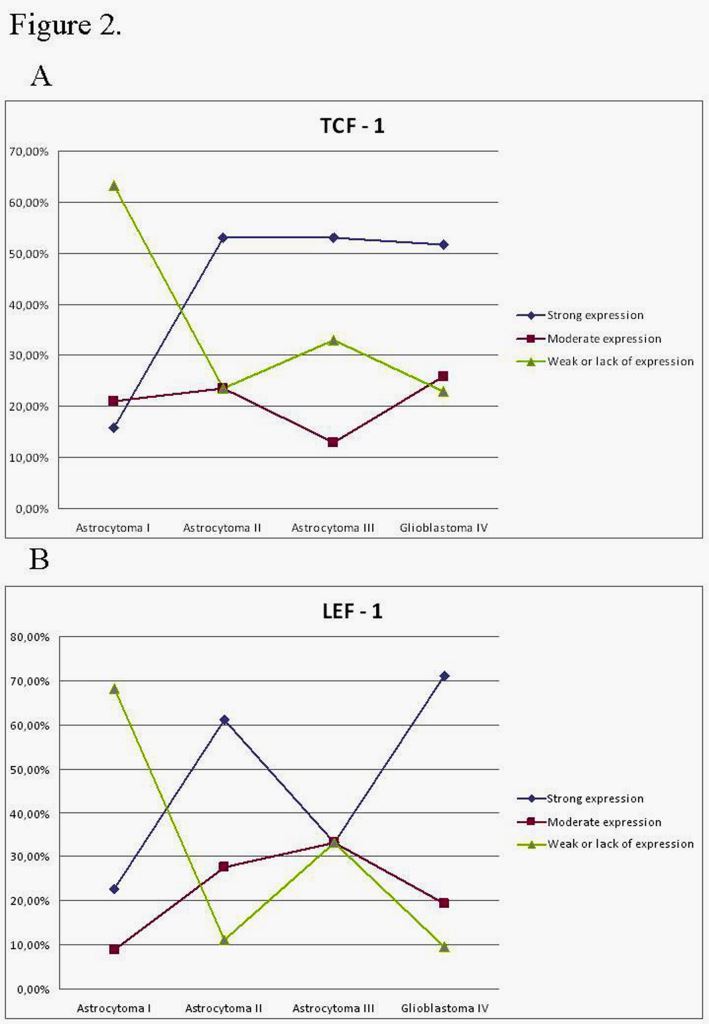
Wnt signaling transcription factors TCF-1 and LEF-1 are upregulated in malignant astrocytic brain tumors
3.Croat Med J 2014, Q2, IF 1,603
The cellular story of dishevelleds
4. Mol Cytogenet 2014, Q2, IF 2.662
Genetic changes observed in a case of adult pilocytic astrocytoma revealed by array CGH analysis
5. Pećina-Šlaus N, Kafka A. Wnt signaling and astrocytic brain tumor. CNS Oncol 2015;4(6):369-70. doi:10.2217/cns.15.24
http://www.futuremedicine.com/doi/abs/10.2217/cns.15.24?url_ver=Z39.88-
6. Pećina-Šlaus N, Kafka A, Varošanec AM, Marković L, Krsnik Ž, Njiric N, Mrak G. Expression patterns of Wnt signaling component sFRP3 in astrocytoma and glioblastoma. Mol Med Rep 2016;13:4245-4251. 10.3892/mmr.2016.5061
https://www.spandidos-publications.com/mmr/13/5/4245
7. Pećina-Šlaus N, Kafka A, Vladušić T, Tomas D, Logara M, Skoko J, Hrašćan R. Loss of p53 expression is accompanied with upregulation of beta-catenin in meningiomas: a concomitant reciprocal expression. Int J Exp Pathol 2016 doi: 10.1111/iep.12186
http://onlinelibrary.wiley.com/wol1/doi/10.1111/iep.12186/full
8. Pećina-Šlaus N, Kafka A. Lechpammer M. Molecular genetics of intracranial meningiomas with emphasis on canonical Wnt signalling. Cancers 2016;8:x. doi:10.3390
http://www.mdpi.com/2072-6694/8/7/67
9. Kafka A, Tomas D, Lechpammer M, Gabud T, Pažanin L, Pećina-Šlaus N. Expression Levels and Localizations of DVL3 and sFRP3 in Glioblastoma. Dis Markers. 2017;2017:9253495.
10. Kafka A, Bačić M, Tomas D, et al. Different behaviour of DVL1, DVL2, DVL3 in astrocytoma malignancy grades and their association to TCF1 and LEF1 upregulation. J Cell Mol Med. 2018;
11. Kafka A, Karin-kujundžić V, Šerman L, et al. Hypermethylation of Secreted Frizzled Related Protein 1 gene promoter in different astrocytoma grades. Croat Med J. 2018;59(5):213-223.
12.
Experimental protocol for IHC (Protokol za IHK)
IMUNOHISTOKEMIJSKA METODA OTKRIVANJA PROTEINA
Među brojnim molekularnobiološkim metodama koje su razvijene u novije vrijeme imunocitokemija i imunohistokemija vrlo su korisne za otkrivanje specifičnih proteina. Tim metodama uz upotrebu monoklonskih protutijela, moguće je ciljano otkriti je li u pojedinoj stanici ili tkivu došlo do ekspresije pojedinih gena, odnosno jesu li geni izrazili proteinske produkte u stanici. Krajnji produkt ekspresije gena koji se ispituje njegov je proteinski produkt. U znanstvenim istraživanjima, ali danas i u dijagnostičke svrhe u patologiji, izuzetno je važno ustanoviti prisutnost, odnosno odsutnost proteinskoga produkta pojedinog gena.
Metoda peroksidaza – antiperoksidaza
Proteinske produkte ispitivanog gena detektirat ćemo s pomoću specifičnih monoklonskih protutijela primjenom metode peroksidaza – antiperoksidaza uz vizualizaciju DAB (3,3′-diamino benzidin tetraklorid) kromogenom. Postupak se izvodi na histološkim preparatima, a započinje deparafinizacijom koju provodimo dvostrukim tretiranjem preparata ksilenom tijekom 5 min. Preparate zatim uranjamo u apsolutni alkohol 3 min, te u 95%-tni alkohol također 3 min (sve Kemika, Zagreb). Odlučili smo opisati metodu koju vrlo dobro provodi komercijalni “kit” tvrtke DAKO. Nakon ispiranja u destiliranoj vodi 30 s, a zbog odstranjivanja viška organskih otapala i dodatne hidracije, slijedi inkubacija u DAKO Target retrieval otopini (DAKO No. S1700) uz zagrijavanje u mikrovalnoj pećnici na 800 W triput tijekom 5 min. Ovaj korak služi oporavljanju epitopa skrivenih ili nestalih tijekom fiksacije i uklapanja. Ulogu oporavljanja epitopa ima citratni pufer, pH=6,0 koji je glavni sastojak DAKO Target retrieval otopine. Nakon zadnjeg 5 minutnog zagrijavanja predmetna stakalca ostavljamo da se 20 min ohlade u Target retrieval otopini. Slijede tri ispiranja u puferu PBS (engl. Phosphate-Buffered Saline) pH 7,2. Preparate zatim tretiramo 3%-tnim H2O2 (DAKO –No.K0679) 5 min, a radi inaktivacije endogene peroksidaze. Ovo je bitan korak jer se njime izbjegava nespecifično oksidiranje DAB kromogena endogenim peroksidazama. Slijedi ispiranje u puferu PBS-u, te uklanjanje nespecifičnog vezanja s mišjim serumom (Imunološki zavod, Zagreb) tijekom 30 min.
Inkubaciju željenim primarnim protutijelom u optimiziranom razrjeđenju, koje je najčešće 1:100 ili 1:200, a koja traje 30 min, slijede tri ispiranja po 5 min u puferu PBS u koji je dodan kozji serum. Preparate zatim inkubiramo sekundarnim LINK protutijelom (DAKO No.K0679) tijekom 25 min. Sekundarno protutijelo prepoznaje Fc fragment primarnog protutijela, veže se na njega i tako ga obilježava. Također, na vlastitom Fc fragmentu sekundarno protutijelo ima povezan biotin što je bitno za kasniju reaktivnost s drugim reagensima.
Ponovno slijedi ispiranje u puferu PBS u koji je dodan kozji serum, a zatim inkubacija streptavidin peroksidazom iz hrena (engl. horseradish; DAKO No.K0679) tijekom 25 min.
Stakalca zatim isperemo u puferu PBS te dodajemo DAB kromogen (3,3′-diamino benzidin tetraklorid, DAKO No.K0679.) i inkubiramo 3 min. Preparate potom isperemo u destiliranoj vodi i bojimo hematoksilinom tijekom 3 min. Ponavljamo postupak ispiranja te ih osušene uklapamo u otopinu pufera PBS i glicerola (omjer 50:50).
Važno je tijekom eksperimenta uvijek imati i negativnu kontrolu koju najčešće čini uzorak koji nismo inkubirali primarnim protutijelom tijekom postupka imunohistokemije. Pozitivna kontrola isto je tako vrlo važna, a to je najčešće tkivo za koje iz znanstvene literature ili vlastita iskustva znamo da eksprimira protein (antigen) koji istražujemo. Rezultate imunohistokemije analiziramo na mikroskopu.
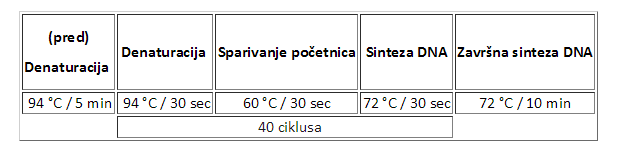
Conditions for PCR reactions (Protokoli za PCR)


Rezultati ekspresije čimbenika transkripcije TCF1 i LEF1
Diseminacijske aktivnosti na projektu:
Nives Pećina-Šlaus; Prezentacija projekta “The role of wnt signaling in epithelial to mesenchymal transition” u sklopu manifestacije Tjedan mozga. 19.03.2015.Zagreb.
Nives Pećina-Šlaus; pozvano predavanje: Prezentacija projekta “Molekularne promjene signalnog puta WNT u astrocitnim tumorima mozga” 24.03. 2015, Zagreb.
Pećina-Šlaus N, Kafka A, Tomas D, Krušlin B; izlagenje na konferenciji: Upregulation of key wnt signaling molecules in human astrocytic brain tumorsThe European Human Genetics Conference, Milano, Italy, May 31-June 3, European Journal of Human Genetics 22 Suppl 1, 265, 2014
Nives Pećina-Šlaus; pozvano predavanje:Molecular changes of Wnt signaling play important roles in astrocytic tumor etiology. TM’s 3rd World Genetics & Genomics Online Conference,May 20-22, 2014.on-line konferencija kojoj mogu pristupiti svi iz svijetahttp://www.nature.com/natureevents/science/events/24755-TM_s_3rd_World_Genetics_Genomics_Online_Conference
Nives Pećina-Šlaus; pozvano predavanje:Prezentacija projekta “Involvement of key components of wnt signalling in human astrocytic brain tumours”14.05.2015.London, UK, EuoSciCon
Anja Kafka, izlaganje na konferenciji; Kafka A, Nikuševa Martić T, Marković L, Varošanec A, Bačić M, Beroš V, Pećina-Šlaus N (2015) Molecular changes of wnt signaling play important roles in astrocytic brain tumor etiology. FEBS, Berlin, Germany, July 4-9, 2015.
Izlaganje na radionici; Kafka A, Tomas D, Marković L, Okštajner PK, Sukser V, Pecina-Slaus N (2014) Wnt transcription factor LEF1 as a potential diagnostic marker of higher grade gliomas. GlowBrain Workshop, Zagreb, Croatia, March 27-29, 2014.
Izlaganje na radionici; Kafka A, Bacic M, Moric M, Skoko J, Pecina-Slaus N. Chganges of central mediators of wnt signaling DVL1 and DVL3 in human glioblastoma. GlowBrain Workshop, Zagreb, Croatia, January 29-31, 2015.
Izlaganje na konferenciji; Kafka A, Pećina-Šaus N (2015) Changes in gene structure and protein expression of DVL1, DVL2, DVL3 and transcription factors TCF1 and LEF1 in glioblastoma. PhD Day, Zagreb, Croatia, May 22, 2015.
Kafka A, Hrašćan R, Vladušić T, Logara M, Skoko J, Varošanec A, Marković L, Tomas D, Pećina-Šlaus N (2015) Reciprocal relationship between beta-catenin and p53 expression in meningioma. 5th Croatian Neuroscience Congress, Split, Croatia, September 17-19, 2015.
Kafka A, Varošanec A, Marković L, Krsnik Ž, Tomas D, Bačić M, Pećina-Šlaus N (2015) Expression patterns of Wnt signaling key components: Dishevelleds, transcription factors TCF/LEF and antagonist SFRP3 in astrocytic brain tumors. 5th Croatian Neuroscience Congress, Split, Croatia, September 17-19, 2015.
Kafka A, Babić Leko M, Njirić N, Logara M, Mrak G, Pećina-Šlaus N (2016) A concomitant reciprocal expression of p53 and beta-catenin in meningiomas. EACR Conference: A matter of life or death. Amsterdam, Netherlands, January 28-30, 2016.
Pećina-Šlaus N, Kafka A, Skoko J, Logara M, Njirić N, Tomas D (2016) Wnt signalling is targeted in meningioma. The controlling cancer summit, London, UK, May 17-19, 2016
Kafka A, Tomas D, Marković L, Varošanec A, Njirić N, Krsnik Ž, Mrak G, Pećina-Šlaus N (2016) A dual role of sFRP3 modulatin molecule in astrocytic brain tumors. ESHG, Barcelona, Spain, May 21-24, 2016.
Rezultati treće godine projekta HRZZ WNT4EMT
Znanstveni radovi objavljeni/u postupku objave tijekom treće godine projekta
Pećina-Šlaus N, Kafka A, Vladušić T, Pećina HI, Hrašćan R. AXIN1’s expression and localization in meningiomas and association to changes of APC and E-cadherin. Anticancer Res 2016;36:4583-94. doi:10.21873/anticanres.11007 IF 1,895, Q3
Pećina-Šlaus N, Kafka A, Bukovac A, Vladušić T, Tomas D, Hrašćan R. Genetic changes of MLH1 and MSH2 genes could explain constant findings on microsatellite instability in intracranial meningioma. Tumor Biol, 39 (7), 1-9, 2017. IF 3,650, Q2, DOI: 10.1177/1010428317705791
Pećina-Šlaus N, Bukovac A, Salomon I, Kafka A. Microsatellite instability as a driving force for cancer progression. Cancer Hypotheses 2017 1, 6, 1-16.
Kardum V, Karin V, Glibo M, Skrtic A, Nikuseva Martic T, Ibisevic N, Skenderi F, Vranic S, Serman Lj. Methylation-associated silencing of SFRP1 gene in high-grade serous ovarian. Annals of Diagnostic Pathology 2017;31:45–49, IF 1,734; Q3
Kafka A, Tomas D, Lechpammer M, Gabud T, Pecina-Slaus N. The relationship of expression levels and localizations of DVL3 and sFRP3 in glioblastoma. Dis Markers 2017 In process of peer review IF 2,348, Q2
Kafka A, Karin V, Šerman Lj, Bukovac A, Njirić N, Jakovčević A, Pećina-Šlaus N. Promoter hypermethylation of Wnt pathway inhibitor sFRP1 gene and its expression levels in human astrocytoma. BMC Cancer 2017 In process of peer review IF 3,288, Q2
Popis diseminacijskih aktivnosti
Pećina-Šlaus N, Kafka A, Tomas D, Marković L, Varošanec AM, Bačić M, Njirić N (2016) Wnt signaling is activated in human astrocytomas. EMBO Conference, Brno, Czech Republic, September 14-17, 2016.
Kafka A, Bukovac A, Tomas D, Lechpammer M, Gabud T, Pećina-Šlaus N (2016) The interplay between DVL3 and sFRP3 in glioblastoma. HDIR-4, 4th meeting of the croatian association for cancer research, Zagreb, Croatia, November 3-4, 2016.
Pećina-Šlaus N, Kafka A, Njirić N, Bukovac A, Tomas D (2016) Key Wnt signalling molecules as potential biomarkers of astrocytic brain tumors. 6th Munich Biomarker Conference. Munich, Germany, November 29-30, 2016.
Brlek P, Horvat Velić E, Bukovac A, Kafka A, Tomas D, Pećina-Šlaus N (2017) Levels of N-cadherin expression in human meningioma. 7th Student Congress of Neuroscience. Rijeka/ Rab, Hrvatska, April 21-23, 2017.
Kafka A, Bukovac A, Brlek P, Bačić M, Pećina-Šlaus N. Detection of microsatellite instability and loss of heterozygosity of DVL1, DVL2 and DVL3 gene in astrocytic brain tumors. 3rd EACR Conference on Cancer Genomics, Cambridge, UK, June 25-28, 2017.
Kafka A, Njirić N, Bačić M, Bukovac A, Tomas D, Mrak G, Pećina-Šlaus N. Changes in gene structure and protein expression of DVL1, DVL2, DVL3 and transcription factors TCF1 and LEF1 in astrocytic brain tumors. EACR-FEBS Advanced Lecture Course: Molecular Mechanism in Signal Transduction in Cancer, Spetses, Greece, August 16-24, 2017.
Pozvana predavanja
Pećina-Šlaus N. Toliko „OMICSa“, a samo jedno zdravlje. 4. Simpozij apoptoza i novotvorine, Knjižnica HAZU, Zagreb, 28.03.2017. http://info.hazu.hr/hr/novosti_i_dogadanja/kalendar_dogadanja/4-simpozij-apoptoza-i-novotvorine,3568.html
Kafka A. Uloga gena i proteina Dishevelled u astrocitnim tumorima mozga. 4. Simpozij apoptoza i novotvorine, Knjižnica HAZU, Zagreb, 28.03.2017. http://info.hazu.hr/hr/novosti_i_dogadanja/kalendar_dogadanja/4-simpozij-apoptoza-i-novotvorine,3568.html
Bukovac A. The impact of cadherin switch on the epithelial-mesenchymal transition and phosphorylation status of beta-catenin in intracranial meningiomas, 21st Young Neuroscientist Meeting 29.06.2017 – 29.06.2017, Zagreb
Ostale objavljene publikacije proizašle iz aktivnosti na projektu
1. Pećina-Šlaus N., Kafka A., Njirić, N. Mitohondrij i mitohondrijske bolesti. Medicinar 58; 47-50, 2016.
2. Pećina-Šlaus N., Kafka A., Njirić, N. Mitohondrij i mitohondrijske bolesti. Sonda 18 (33), 81-84, 2017.
3. Pećina-Šlaus N. Bakterijski sustav CRISPR/Cas9: oruđe precizne modifikacije genoma. Mef. List Medicinskog fakulteta, Prosinac 2016 / Godina 35, br. 2
4. Pecina-Šlaus N. Konferencija Cancer Genomics, Mef. srpanj 2017, godina 36, br.1 str. 95
Odobren nam je projekt 6. 2016.-2020. „Razvoj karijere mladih znanstvenika“, HRZZ (voditelj –mentor Nives Pećina-Šlaus).
Dokumenti