Naziv projekta: IP-2014-09-4173 Reprogramiranje citoprotektivnih puteva u mezoteliomu (Reprogramming cytoprotective pathways in mesothelioma)
Funding agency: Hrvatska zaklada za znanost
Principal investigator: prof. dr. sc. Sven Seiwerth
Duration: 4 years
Budget: 1.000.000,00 HRK
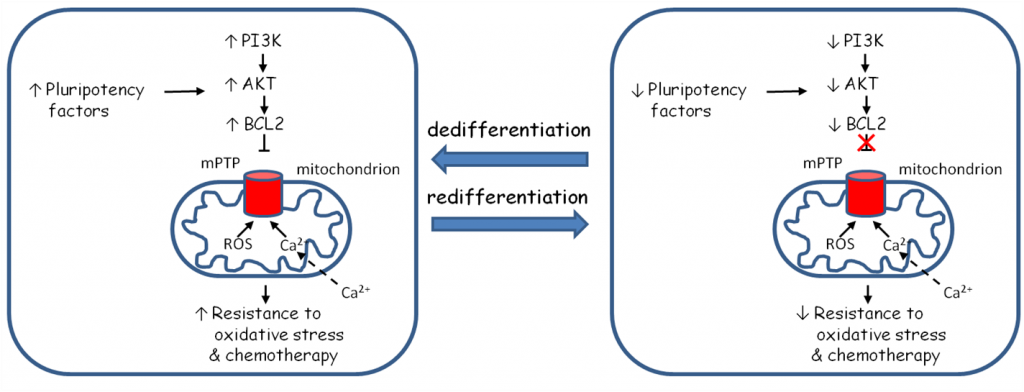
Overall hypothesis. Reprogramming mesothelioma cells modifies regulatory pathway of the mitochondrial permeability transition pore (mPTP), which translates to altered response to chemotherapeutic agents and oxidative stress.
INFORMACIJE O PROJEKTU
It is generally believed that the evolution of a cancer is a stochastic process of mutation-induced activation and inactivation of principal genes by which tumor cells acquire phenotypic advantages over normal cells allowing them to proliferate and migrate locally and to remote organs. Recent data indicate that some of key features of malignancy are similar to normal processes of embryonic development, which are programmed and highly regulated. The subpopulation of cancer cells that exhibit properties of embryonic stem cells are called cancer stem cells (CSCs) and studies showed their essential role in resistance to radio- and chemotherapy, tumor dormancy and metastasizing. We aim to investigate malignant characteristics of mesothelioma that are driven by programmed processes associated with the regulation of pluripotency, and which can be modified by exogenous treatment. Our overall hypothesis is that the resistance to oxidative stress and chemotherapeutics in mesothelioma cells is regulated by the activity of oncogenic pathway PI3K/AKT/BCL2. It will be investigated whether this pathway can be controlled with master regulators of pluripotency and differentiation by reprogramming mesothelioma cells. Mesothelioma cells will be reprogrammed to pluripotent state with introduction of defined factors yielding induced pluripotent mesothelioma stem cells (iPMSCs). iPMSCs will be differentiated into three different lineages. This will allow us to determine whether such experimental perturbation decreases activity of the specific oncogenic pathway PI3K/AKT/BCL2 that may control mitochondrial cytoprotective pathways. Ultimately such intervention may render cells more sensitive to oxidative stress and chemotherapeutics. In vitro studies will be corroborated with clinically relevant histopathological examination of patient’s tumors where correlation among differentiation status, PI3K/AKT/BCL2 pathway activity and patient’s survival will be examined.
SUDIONICI PROJEKTA
Filip Sedlić
School of Medicine Zagreb, Croatia
Ana Šepac
School of Medicine Zagreb, Croatia
Luka Brčić
School of Medicine Zagreb, Croatia
Iva Brčić
Clinical Medical Center Zagreb, Croatia
Marija Mišić
School of Medicine Zagreb, Croatia
Kristina Meljanac
School of Medicine Zagreb, Croatia
Mir Ali Reza Hoda
Medical University of Vienna, Austria
Dome Balazs
Medical University of Vienna, Austria
Marko Jakopović
School of Medicine Zagreb, Croatia