Diseminacija i vidljivost
Radionica - International Workshop Design and Implementation of Inclusive Clinical Studies
Over 50 stakeholders actively participated in the International Workshop Design and Implementation of Inclusive Clinical Studies, organised by SHINE 2Europe and held online on 21st January 2025. The event explored this topic within three Horizon Europe projects focused on cancer and cardiovascular research – RadioVal, Liveration and AI4HF - and one in the frame of the Innovative Medicine Initiative, REaDI, all in which clinical studies and trials play a central role. Designing inclusive clinical studies is often challenging due to time, human, and financial constraints, enhanced by scattered legal and regulatory frameworks, and challenges in uniform data collection. All of these issues tend to overload the professionals developing clinical studies, but overlooking inclusivity poses an even greater risk: the exclusion of underrepresented groups from research and even from optimal medical care. During the workshop, a wide audience – including researchers, healthcare professionals, AI and IT developers, ethicists, regulators, policymakers, social scientists and patients – analised the main challenges to achieve more inclusive clinical studies, potential solutions and the relevant stakeholders involved in each.
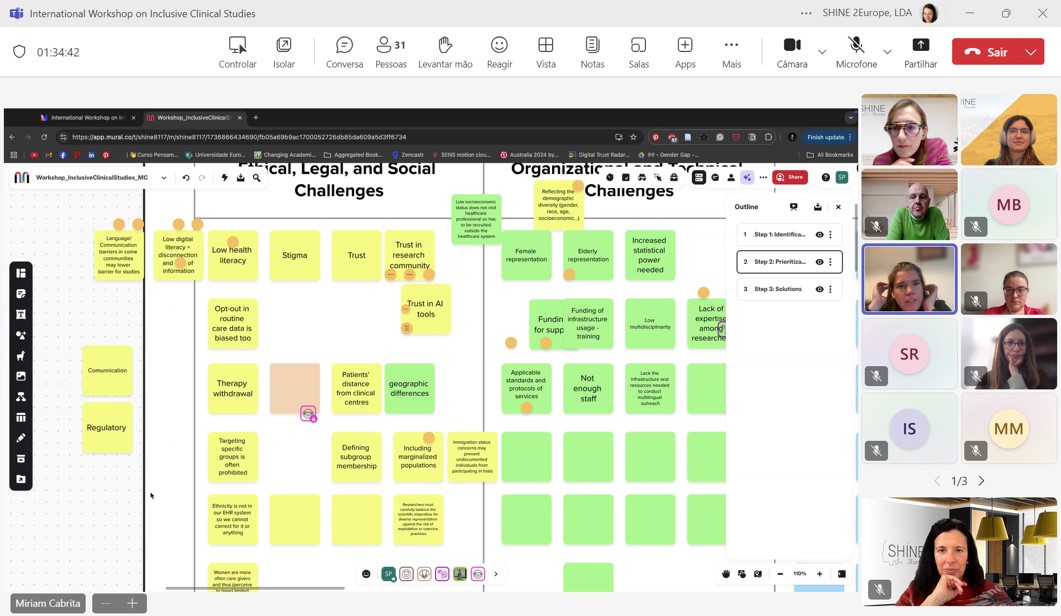
Four high-level speakers presented the featured projects, focusing on the clinical studies aspects, and providing insights into how aspects of diversity, equity and inclusion have been considered in the design and will be further addressed in the implementation of such studies. The group of participants was then divided into three break-out rooms to discuss in-depth the challenges for inclusive clinical studies, divided into different domains, such as ethical, legal, social, technical, organisational and others. Then, in each group, the challenges were prioritised and the one considered as most pressing was further analised in terms of potential solutions and the relevant actors that can enable such results. During the discussion, several challenges emerged, such as:
1. Literacy, namely health, digital and traditional literacy
2. Diversity, equity, and inclusion training for all stakeholders involved in the design and implementation of clinical studies, especially for clinical and research teams
3. More flexibility in the application of a study protocol, to encourage inclusion
4. Access, on a geographical base, as trials are often conducted in urban or academic centres, limiting access for rural (or smaller towns) populations
A very urgent challenge identified was also the limited time and funding available for trials and clinical studies, which significantly impacts their implementation. Proposed solutions included calling on the European Commission to design calls for proposals that better address the specific needs of trials, promoting greater collaboration to overcome organisational barriers and using innovative digital tools along with comprehensive routine data collection for patient selection, thereby ensuring greater equity.
The outcomes of this workshop will be further analysed and shared very soon. The detailed agenda and pictures of the session follow attached.
RadioVal: ECR 2025 - Invitation to attend or watch RadioVal presentation
Our project will be featured at ECR 2025 in a session titled "Intelligence at Work: Harnessing AI for Clinical Decision-Making" on March 1, from 16:00 to 17:30.
Prof. Karim Lekadir and Gianna Tsakou will represent our project at this important event, and I highly encourage you to attend if you are available. Alternatively, you can watch the session online by registering through the following link.